
Answer to Give the MO diagram for Cl2 Question: Give the MO diagram for Cl2. Give the MO diagram for Cl2.

see more. Expert Answer.

% (2 ratings). Click here to get an answer to your question ✍ how to drew molecular orbital diagram of Cl2.

Cl2 molecular orbital diagram. In contrast to crystal field theory, molecular orbital included the covalent nature of the metal-ligand bond interaction.
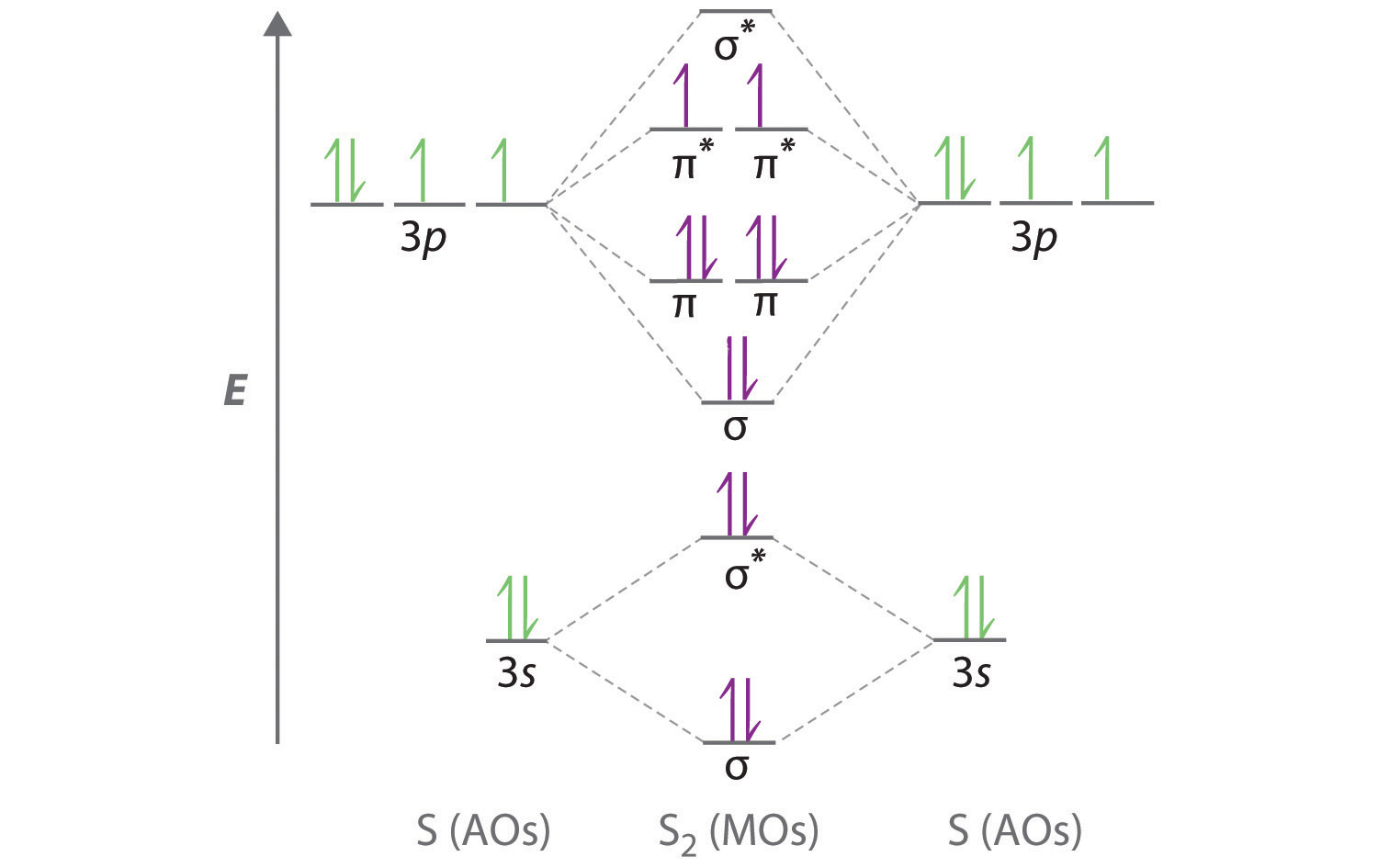
Energy of. Cl atom has 17 electrons, so chlorine molecule has (Cl2) has 34 electrons. so, bond order of chlorine molecule is 1.

How do I determine the bond order using the Lewis structure?. The Lewis dot structure famously predicts the wrong electronic structure for O2.

• We can use LCAO-MO theory to get a better picture: 2sa. 2pa.MO Diagram of I 2 MO Diagram of I 2-Base Complex In this experiment we analyze the acid-base interaction by comparing the energies of the I2 transition to that of the donor-acceptor transition.

The higher the energy the absorption, the stronger the acid-base interaction. MOLECULAR ORBITAL DIAGRAM KEY Draw molecular orbital diagrams for each of the following molecules or ions.
Determine the bond order of each and use this to predict the stability of the bond.

Determine whether each is paramagnetic or diamagnetic. a.

H 2 B.O. = 1 stable diamagnetic b.

He 2 B.O. = 0 unstable diamagnetic σ 1s ∗ σ 1s σ 1s.
eV. Because of the difference in their atomic orbital energies, the 1s orbital of hydrogen and the 3s orbital of sulfur interact only weakly; this is shown in the diagram by a slight stabilization of the lowest energy molecular orbital with respect to the 3s orbital of sulfur.

This lowest energy orbital is . Chapter 6 – Chemical Bonding * Diatomic Molecules & Lewis Structures – Diatomic molecules include: H 2, N2, O2, F 2, Cl 2, Br 2, or I2 – Lewis proposed that electrons are shared between neighboring atoms and thereby – Helium Molecular Orbital Diagram * 1 1 2 1 1 s s s s He. MOLECULAR ORBITAL ENERGY LEVEL DIAGRAMS Figure shows molecular orbital energy level diagrams for homonuclear diatomic mole-cules of elements in the first and second periods.

Each diagram is an extension of the right-hand diagram in Figure , to which we have added the molecular orbitals formed from 2s and 2p atomic orbitals.Solution: Consider the species Cl2+, Cl2, | ChemistryMolecular Orbital Diagrams of Diatomic Molecules – Chem